Strategic Sourcing for Pharmacovigilance (PV) Services – Creating a Collaborative Environment to Improve Services and Reduce Costs
Sourcing is often designed as a cost saving exercise. However, in the case of complex services that are integral to an enterprise success, sourcing can often result in a better solution for the organization, not just savings. We like to think of this as a win-win opportunity – better service levels, lower costs – and if driven/supported by senior leadership can reap great rewards. Below I share an excerpt from Dave Stowe, a long-time member of our OnDemand Professional Network, that I think exemplifies this kind of complex sourcing – this time at a high-growth BioPharma company that was outgrowing the capabilities of it’s Pharmacovigilance Services (PV*) provider.
SITUATION OVERVIEW
A rapidly growing $1.3B biopharma company with 12 drugs on the market and ongoing drug development, needed to find a new supplier for PV services. As a result of the company’s rapid growth over the preceding 8 years, the incumbent supplier was unable to meet service level requirements creating a sizable business risk. The client had increasing concerns about the incumbent’s capability to respond to audits in a timely manner; rapidly scale to support spikes in volumes; and support advanced analytics and insights. As a result, executives including the EVP of R&D, CFO and CEO approved a sourcing effort to find a new service supplier and technology platform.
To lead the sourcing effort, the client brought in Dave Stowe, a member of the OnDemand Professional Network with deep pharmaceutical industry sourcing expertise to work with the VP of Patient Safety, scientists and the IT organization.

APPROACH
The client created a core team with representatives from PV, IT and procurement to manage the details of the sourcing process and established a steering committee to provide executive oversight prior to making the final recommendation.
The core team developed a three phased approach starting with a Request-for-information (RFI), continuing with a Request-for-Proposal (RFP) and concluding with a recommended vendor and technology platform for executive approval. This approach provided them with an opportunity to thoroughly understand the supply market, services, technology platforms and cost structure.
For the RFI phase, the team engaged nine suppliers to solicit information on services and technology platforms on how to deploy business processes to meet requirements for:
- Case processing
- Aggregate safety data reporting
- Literature services
- Signal detection and management
- Data visualization and analytics
- Quality metrics and inspections
As a result of the knowledge gained through the RFI phase, the team was able to select a technology platform, develop an RFP and down select four suppliers to solicit formal proposals.
To evaluate the proposals, the team:
- Developed a comprehensive set of business and financial criteria
- Solicited input from stakeholders across the R&D, regulatory, quality, medical information, regulatory affairs, legal and clinical operations to meet with the supplier teams and evaluate their offerings.
CRITICAL SUCCESS FACTORS
- Facilitating working meetings with stakeholders to accurately capture requirements and issues across the following four areas:
- Incumbent performance
- Technology platform requirements
- Service provider requirements
- Transition requirements
- Creating a collaborative RFI/RFP process with suppliers to uncover options which would serve the client team vs. standard offerings
Establishing and defining the roles for the following 3 different stakeholders:
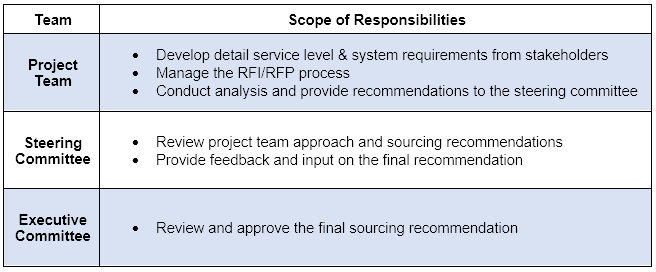
As a result of this collaborative approach, the team was able to develop a solution acceptable to the users, finance, legal and the executive committee.
RESULTS
The engagement generated a 36% reduction (savings) in run rate costs for both the services and technology platform. Accounting for one-time transition costs to the new supplier and technology platform, the project generated 19% in overall cost savings.
Solution provided the PV team with a leading best in class technology platform and provided out of the box ability to integrate with company’s clinical trial system. Platform provided extensive analytic and reporting capabilities and continuous updates compliant with worldwide regulatory standards for PV case management.
Finally, the sourcing analysis was “executive ready” to secure agreement from the CEO and executive committed to finalize the contract with a new supplier.

OnDemand Professional Network Member Since 2005
Dave works with clients to create value through strong supplier relationships. He has developed and implemented procurement organization models bringing together organizational responsibilities, policies, systems and metrics in both industry leadership and consulting roles. In addition, he has sourced global goods and services for multiple direct and indirect categories in life sciences, healthcare, aerospace and industrial products. Dave earned his MBA and a MS Industrial Engineering from the University of Michigan and a BS Industrial Management from Purdue University.




Tom Donatelli Joins OnDemand To Enhance Our Client Service Capabilities And Broaden Our Market Reach